What is the solvent effect?
The solvent effect is the influence of the solvent on the physical and chemical properties of a solute. Solvents can affect a wide range of properties, including solubility, reactivity, stability, and spectroscopy.
There are a number of different mechanisms by which solvents can exert their effects. One common mechanism is through solvation, in which the solvent molecules interact with the solute molecules. This can lead to changes in the solute’s structure, polarity, and electronic properties.
Another common mechanism is through hydrogen bonding. Hydrogen bonding can occur between the solvent molecules and the solute molecules, or between two or more solvent molecules themselves. Hydrogen bonding can also lead to changes in the solute’s structure, polarity, and electronic properties.
Other mechanisms of solvent effects include:
- Ion-dipole interactions: These interactions occur between ions in the solvent and polar molecules or charged groups in the solute.
- Dipole-dipole interactions: These interactions occur between polar molecules in the solvent and polar molecules or charged groups in the solute.
- London dispersion forces: These weak attractive forces occur between all molecules and atoms, regardless of their polarity.
Impact on Reaction Kinetics and Equilibria
The solvent can have a significant impact on the kinetics and equilibria of chemical reactions. For example, a solvent can change the activation energy of a reaction, which can affect the rate of the reaction. A solvent can also change the equilibrium constant of a reaction , which can affect the distribution of products and reactants at equilibrium.
Examples of Solvent Effects in Various Chemical Processes
Solvent effects are important in a wide range of chemical processes, including:
- Organic synthesis: Solvents are used to dissolve reactants, products, and catalysts in organic synthesis reactions. The solvent can have a significant impact on the yield, selectivity, and purity of the products.
- Catalysis: Solvents can play an important role in catalysis by affecting the activity and selectivity of catalysts.
- Electrochemistry: Solvents are used in electrochemical cells to dissolve electrolytes and to transport ions. The solvent can affect the conductivity of the electrolyte and the potential of the electrodes.
- Analytical chemistry: Solvents are used in a variety of analytical techniques, such as chromatography and spectroscopy. The solvent can affect the solubility of the sample and the resolution of the analysis.
Characterization of solvent effect
The solvent effect can be characterized by the following:
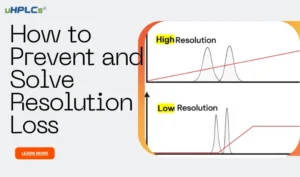
- Peak shape abnormalities: This is the most intuitive and common manifestation of solvent effects. Examples include peak bifurcation, trailing, and leading edge.
- Varying retention times and unstable response values: These are also common manifestations and may be accompanied by abnormal peak shapes.
- Normal peaks with large fluctuations in response values: This is a more difficult-to-detect type of solvent effect that can be easily misjudged as a result of instrument failure or other system applicability issues.
Other cases not listed in this paper can also be evaluated for the risk of solvent effect according to the way of thinking in this paper.
In addition to the above, the solvent effect can also be characterized by the following changes in the chromatographic parameters:
- Column efficiency: The solvent effect can reduce column efficiency, leading to broader peaks and lower resolution.
- Selectivity: The solvent effect can change the selectivity of the stationary phase, leading to different elution orders for the components in the sample.
- Capacity factor: The solvent effect can change the capacity factor of the components in the sample, leading to different retention times.
- Peak height: The solvent effect can decrease the peak height of the components in the sample, leading to lower response values.
The solvent effect can also be characterized by the following changes in the physical properties of the sample:
- Solubility: The solvent effect can affect the solubility of the components in the sample, leading to different peak areas.
- Ionization state: The solvent effect can affect the ionization state of the components in the sample, leading to different retention times and response values.
- Aggregation: The solvent effect can affect the aggregation of the components in the sample, leading to different peak shapes and retention times.
By understanding the characteristics of the solvent effect, chromatographers can take steps to minimize its impact on their analyses. For example, they can choose a different diluent or mobile phase, adjust the pH of the mobile phase, or use a different column packing.
How Selection of Appropriate Solvents
Criteria for Solvent Selection to Minimize Negative Solvent Effects
When selecting solvents for HPLC analysis, it is important to consider the potential for negative solvent effects. The following criteria can be used to select solvents that minimize negative solvent effects:
- Avoid solvents with high polarity: Polar solvents can interact strongly with the solute and the column packing, which can lead to peak broadening and other negative solvent effects.
- Choose solvents with a dielectric constant similar to that of the mobile phase: This will help to minimize the difference in solvation between the diluent and the mobile phase, which can reduce the risk of solvent effects.
- Avoid solvents with a strong affinity for the solute or the column packing: This will also help to reduce the risk of solvent effects.
- Use a mixture of solvents to reduce the polarity of the mobile phase: This can be useful for solutes that are strongly retained on the column.
- Use a different column packing: Some column packings are more resistant to solvent effects than others.
Considerations of Solvent Polarity, Dielectric Constant, and Solvent Structure
The polarity of a solvent is a measure of its ability to interact with other polar molecules. Polar solvents have a dipole moment, which means that they have a positive end and a negative end. Nonpolar solvents do not have a dipole moment.
The dielectric constant of a solvent is a measure of its ability to weaken the electrostatic forces between charged particles. Solvents with a high dielectric constant are better at solvating ions than solvents with a low dielectric constant.
The structure of a solvent can also affect its polarity and dielectric constant. For example, solvents with hydrogen bonding groups, such as water and alcohols, are more polar than solvents without hydrogen bonding groups, such as hydrocarbons.
Solvent Screening Methodologies
There are a number of solvent screening methodologies that can be used to select solvents that minimize negative solvent effects. One common approach is to use a trial-and-error approach. This involves injecting the sample into the HPLC system with different solvents and mobile phase compositions until a suitable solvent system is found.
Another approach is to use a solvent selection software. This software can be used to predict the polarity and dielectric constant of different solvents and mobile phase compositions. The software can also be used to predict the retention time of the solute in different solvent systems.
Once a suitable solvent system has been selected, it is important to optimize the system to minimize negative solvent effects. This may involve adjusting the pH of the mobile phase, adding a modifier solvent, or changing the column packing.
By carefully selecting the solvents and optimizing the mobile phase composition, chromatographers can minimize negative solvent effects and improve the accuracy and reproducibility of their analyses.
Solvent Effects on Reaction Mechanism
Solvents can have a significant impact on the mechanism of a chemical reaction. This is because solvents can interact with the transition state and reaction intermediates, which can change the activation energy of the reaction and the overall reaction pathway.
One way that solvents can affect the reaction mechanism is by changing the stability of the transition state. For example, a solvent can stabilize the transition state by solvating the charged or polar groups in the transition state. This can reduce the activation energy of the reaction and make the reaction faster.
Another way that solvents can affect the reaction mechanism is by changing the reactivity of the reaction intermediates. For example, a solvent can protonate or deprotonate a reaction intermediate, which can change its reactivity.
Case Studies Where Solvent Choice Has Modified the Reaction Pathway
Here are some examples of how solvent choice can modify the reaction pathway:
- The SN1 reaction: The SN1 reaction is a substitution reaction in which a nucleophile attacks a carbocation intermediate. The rate of the SN1 reaction is dependent on the stability of the carbocation intermediate. Solvents that can stabilize the carbocation intermediate, such as protic solvents, will accelerate the SN1 reaction.
- The E1 reaction: The E1 reaction is an elimination reaction in which a proton is removed from a carbon atom, followed by the elimination of a leaving group. The rate of the E1 reaction is dependent on the stability of the carbocation intermediate. Solvents that can Stabilize the carbocation intermediate, such as protic solvents, will accelerate the E1 reaction.
- The Claisen rearrangement: The Claisen rearrangement is a rearrangement reaction in which an allyl vinyl ether is rearranged to a γ,δ-unsaturated carbonyl compound. The mechanism of the Claisen rearrangement is dependent on the solvent. In protic solvents, the Claisen rearrangement proceeds through a proton-catalyzed mechanism. In aprotic solvents, the Claisen rearrangement proceeds through a thermal mechanism.
- The Diels-Alder reaction: The Diels-Alder reaction is a cycloaddition reaction between a diene and a dienophile. The Diels-Alder reaction can proceed through either a concerted or a stepwise mechanism. The solvent can affect the mechanism of the Diels-Alder reaction . In polar solvents, the Diels-Alder reaction proceeds through a concerted mechanism. In nonpolar solvents, the Diels-Alder reaction proceeds through a stepwise mechanism.
These are just a few examples of how solvent choice can modify the reaction pathway. By understanding the role of solvents in chemical reactions, chemists can develop better synthetic methods and control the selectivity of chemical reactions.
How Optimizing Solvent Concentrations
To optimize solvent concentrations, it is important to balance the reactants and solvent volumes. The solvent should be present in excess to ensure that the reactants are completely dissolved and that the reaction can proceed to completion. However, too much solvent can also be detrimental, as it can dilute the reactants and reduce the yield of the product.
Balancing Reactants and Solvent Volumes
There are a number of ways to balance reactants and solvent volumes. One simple approach is to use a stoichiometric ratio of reactants. This means that the reactants are added to the reaction in the same ratio as they are consumed in the reaction. For example, if a reaction requires 1 mole of reactant A and 1 mole of reactant B, then 1 mole of each reactant would be added to the reaction.
Another approach to balancing reactants and solvent volumes is to use a limiting reagent. The limiting reagent is the reactant that is consumed first in the reaction. Once the limiting reagent is consumed, the reaction will stop, even if there is still excess of the other reactants. This means that the amount of solvent used in the reaction can be reduced by using a limiting reagent.
Techniques for Determining the Optimal Solvent-to-Solute Ratio
There are a number of techniques that can be used to determine the optimal solvent-to-solute ratio. One common approach is to use a solubility test. This involves dissolving a small amount of the solute in different volumes of solvent until a solution is formed . The optimal solvent-to-solute ratio is the smallest volume of solvent that is required to dissolve the solute.
Another approach to determining the optimal solvent-to-solute ratio is to use a reaction test. This involves running the reaction at different solvent-to-solute ratios and measuring the yield of the product. The optimal solvent-to-solute ratio is the ratio that gives the highest yield of the product.
Once the optimal solvent-to-solute ratio has been determined, it is important to use this ratio consistently to ensure that the reaction is reproducible.
Here are some additional tips for optimizing solvent concentrations:
- Use a solvent that is compatible with the reactants and the reaction conditions.
- Use a solvent that is pure and free of impurities.
- Use a dry solvent if necessary.
- Add the solvent slowly and with stirring to ensure that the reactants are completely dissolved.
- Monitor the reaction and add more solvent if necessary.
- Remove the solvent from the product mixture using an appropriate method, such as distillation or evaporation.
By following these tips, chemists can optimize solvent concentrations and improve the efficiency and reproducibility of their synthetic reactions.
Temperature Control and Solvent Effects
Temperature has a significant effect on solvent behavior. As temperature increases, the solvent molecules become more mobile and the solvent becomes less viscos. This can lead to increased solubility, faster reaction rates, and changes in the selectivity of chemical reactions.
Effect of Temperature on Solvent Behavior
Here are some specific examples of the effect of temperature on solvent behavior:
- Solubility: Solubility increases generally with temperature. This is because the increased mobility of the solvent molecules allows them to better solvate the solute molecules.
- Reaction rates: Reaction rates generally increase with temperature. This is because the increased energy of the solvent molecules allows them to better overcome the activation energy of the reaction.
- Selectivity: The selectivity of a reaction can change with temperature. This is because the relative rates of competing reactions can change with temperature.
Strategies for Temperature Regulation to Mitigate Solvent Effects
There are a number of strategies that can be used to regulate temperature and mitigate solvent effects. These strategies include:
- Using a jacketed reactor: A jacketed reactor is a reactor that has a jacket around it that can be filled with a coolant or heating fluid. This allows for precise control of the temperature of the reaction.
- Using a reflux condenser: A reflux condenser is a device that is used to condense the vaporized solvent and return it to the reactor. This allows for the reaction to be run at a constant temperature, even if the solvent is volatile.
- Using a cryogenic bath: A cryogenic bath is a bath that is filled with a liquid that is at a very low temperature, such as liquid nitrogen or liquid carbon dioxide. This can be used to cool the reaction to a very low temperature.
The best strategy for temperature regulation will depend on the specific reaction and the desired outcome. By carefully controlling the temperature of the reaction, chemists can minimize solvent effects and improve the yield, selectivity, and reproducibility of their reactions.
Here are some additional tips for mitigating solvent effects:
- Use the smallest possible sample volume.
- Use a diluent that is similar to the mobile phase in terms of elution capacity and ionization strength.
- Filter the diluent and mobile phase to remove any impurities.
- Use a column packing that is compatible with the test article.
- Use a detector that is not sensitive to the solvent.
By following these tips, chemists can reduce solvent effects and improve the accuracy and reproducibility of their analyses.
Advanced Techniques to Counteract Solvent Effects
Here are some advanced techniques to counteract solvent effects:
Use of Supercritical Fluids
Supercritical fluids are fluids that are above their critical temperature and pressure. At their critical point, the distinction between the liquid and gas phases disappears. Supercritical fluids have unique properties that make them useful for a variety of applications, including solvent extraction, chemical synthesis, and catalysis.
Supercritical fluids have a number of advantages over traditional solvents. They are more efficient at dissolving solutes, they have lower viscosities, and they are less likely to form azeotropes. This makes them ideal for applications where traditional solvents are not suitable, such as the extraction of natural products and the synthesis of fine chemicals.
Supercritical fluids can also be used to counteract solvent effects in chemical reactions. For example, supercritical carbon dioxide can be used to accelerate the rate of reactions that are limited by diffusion. Supercritical carbon dioxide can also be used to suppress side reactions and improve the selectivity of reactions.
Solvent-Free Reactions
Solvent-free reactions are reactions that are carried out without the use of a solvent. This can be done by using solid-supported reagents or by using molten salts as solvents.
Solid-supported reagents are reagents that are attached to a solid support, such as silica gel or a polymer. This allows the reagents to be easily separated from the products after the reaction is complete.
Molten salts are salts that have been melted. Molten salts have a number of advantages as solvents for chemical reactions. They are non-flammable, they have high thermal stability, and they can be used to dissolve a wide range of solutes.
Solvent-free reactions have a number of advantages over traditional solvent-based reactions. They are more environmentally friendly, they are often more efficient, and they can be used to synthesize products that are difficult to synthesize using traditional methods.
Techniques such as Microwave-Assisted Synthesis and Ultrasonication
Microwave-assisted synthesis (MAS) is a technique that uses microwave radiation to heat and accelerate chemical reactions. MAS has a number of advantages over traditional heating methods. It is faster, more efficient, and more selective.
MAS can be used to counteract solvent effects in chemical reactions. For example, MAS can be used to reduce the amount of solvent required for a reaction. MAS can also be used to suppress side reactions and improve the selectivity of reactions.
Ultrasonication is a technique that uses high-frequency sound waves to create cavitation bubbles in a liquid. These bubbles collapse with great force, which can be used to disrupt aggregates and promote chemical reactions.
Ultrasonication can be used to counteract solvent effects in chemical reactions by improving the mass transfer of reactants and products. Ultrasonication can also be used to initiate reactions that are difficult to initiate using traditional methods.
These are just a few examples of advanced techniques that can be used to counteract solvent effects. By using these techniques, chemists can improve the yield, selectivity, and efficiency of their chemical reactions.
Computational Approaches to Predict Solvent Effects
Computational approaches can be used to predict solvent effects on chemical reactions. These approaches can be used to inform solvent choice and reaction conditions.
Overview of Computational Models and Simulations
Computational models and simulations can be used to predict the properties of solvents and their interactions with solutes. These models can be used to calculate the solubility of solutes, the rates of chemical reactions, and the selectivity of chemical reactions.
One common type of computational model is the molecular dynamics simulation. Molecular dynamics simulations simulate the movement of individual atoms and molecules over time. This allows researchers to study the interactions between solvents and solutes and how these interactions affect the properties of solutions.
Another common type of computational model is the quantum mechanics calculation. Quantum mechanics calculations can be used to calculate the electronic structure of molecules and solvents. This information can be used to predict the reactivity of molecules in different solvents.
How In Silico Studies Can Inform Solvent Choice and Reaction Conditions
In silico studies can be used to inform solvent choice and reaction conditions in a number of ways. For example, computational models can be used to:
- Identify solvents that are likely to dissolve the desired product.
- Predict the rates of chemical reactions in different solvents.
- Identify solvents that are likely to suppress side reactions.
- Optimize the reaction conditions for a given solvent.
Computational approaches can also be used to design new solvents with specific properties. For example, researchers have used computational approaches to design solvents that are more efficient at dissolving biomass or that are more selective for certain types of chemical reactions.
Here are some examples of how computational approaches have been used to inform solvent choice and reaction conditions:
- Researchers have used computational models to identify solvents that are likely to dissolve carbon dioxide. This information has been used to develop new methods for carbon capture and storage.
- Researchers have used computational models to predict the rates of chemical reactions in different solvents. This information has been used to develop new synthetic methods for fine chemicals and pharmaceuticals.
- Researchers have used computational models to identify solvents that are likely to suppress side reactions in organic synthesis. This information has been used to improve the yield and selectivity of chemical reactions.
- Researchers have used computational models to optimize the reaction conditions for a given solvent. This information has been used to develop new methods for the production of biofuels and other renewable chemicals.
- Researchers have used computational approaches to design new solvents for the extraction of natural products. These new solvents are more efficient and environmentally friendly than traditional solvents.
Computational approaches are a powerful tool for predicting and understanding solvent effects. By using these approaches, chemists can develop better synthetic methods, improve the efficiency of chemical processes, and design new solvents with specific properties.
Case Studies: Success Stories in Solvent Effect Management
Here are some case studies where addressing the solvent effect improved reaction outcomes:
Case Study 1: Solvent-Free Synthesis of Pharmaceuticals
Challenge: The synthesis of pharmaceuticals often involves the use of large quantities of solvents. These solvents can be expensive, toxic, and flammable. They can also contribute to environmental pollution.
Solution: Scientists have developed solvent-free methods for the synthesis of pharmaceuticals. These methods use solid-supported reagents or molten salts as solvents.
Result: Solvent-free synthesis methods have reduced the environmental impact of pharmaceutical manufacturing and have improved the efficiency of some synthetic processes.
Case Study 2: Microwave-Assisted Synthesis of Fine Chemicals
Challenge: The synthesis of fine chemicals often requires long reaction times and high temperatures. This can lead to low yields and the formation of unwanted side products.
Solution: Scientists have developed microwave-assisted synthesis methods for the synthesis of fine chemicals. Microwave-assisted synthesis uses microwave radiation to heat and accelerate chemical reactions.
Result: Microwave-assisted synthesis has reduced the reaction times and temperatures required for the synthesis of fine chemicals. This has improved the yields and selectivity of chemical reactions.
Case Study 3: Ultrasonication for the Extraction of Natural Products
Challenge: The extraction of natural products from plants and other biological materials can be difficult and time-consuming. Traditional extraction methods often involve the use of toxic solvents and high temperatures.
Solution: Scientists have developed ultrasonication methods for the extraction of natural products. Ultrasonication uses high-frequency sound waves to create cavitation bubbles in a liquid. The collapse of these bubbles can disrupt plant cells and release natural products.
Result: Ultrasonication has reduced the extraction times and solvent requirements for the extraction of natural products. This has improved the efficiency and environmental friendliness of natural product extraction processes.
Case Study 4: Computational Design of Solvents for Carbon Capture and Storage
Challenge: Carbon capture and storage (CCS) is a technology that can be used to reduce greenhouse gas emissions. CCS involves capturing carbon dioxide from industrial sources and storing it in geological formations.
Solution: Scientists have used computational approaches to design new solvents for CCS. These solvents are more efficient at dissolving carbon dioxide than traditional solvents.
Result: The new solvents have improved the efficiency and cost-effectiveness of CCS.
Lessons Learned
These case studies show that addressing the solvent effect can lead to significant improvements in reaction outcomes. By using solvent-free synthesis methods, microwave-assisted synthesis, ultrasonication, and computational design, scientists have developed new and improved methods for the synthesis of pharmaceuticals, fine chemicals, and natural products. They have also developed new solvents for CCS.
The lessons learned from these case studies can be applied to other areas of chemistry, such as catalysis and materials science. By carefully considering the solvent effect and using appropriate solvent management strategies, chemists can improve the efficiency, selectivity, and sustainability of chemical processes.
Choose HPLC Solvent Filter from uHPLCs
In the realm of high-performance liquid chromatography (HPLC), the purity of solvents is paramount for obtaining reliable and reproducible results. Recognizing this critical need, uHPLCs stands at the forefront as a manufacturer of top-tier solvent filters designed for the most demanding laboratory environments. Our solvent filters are engineered to provide unmatched filtration efficacy, ensuring that your HPLC systems operate with solvents free of contaminants that could otherwise compromise your analytical results.
Features of HPLC Solvent Filter by uHPLCs:
Exceptional Filtration Efficiency: Our filters are designed to remove impurities down to sub-micron levels, maintaining the integrity of your solvents and thereby your chromatographic results.
High Durability and Chemical Compatibility: Constructed with materials that withstand a wide range of solvents, our filters promise durability and resistance to chemical degradation over time.
User-Friendly Design: The solvent filters are crafted for ease of use, ensuring a seamless integration into existing HPLC setups without the need for complex modifications or tools.
Low Extractables and Low Binding: With minimal extractables and low-binding materials, the filters ensure that no additional contaminants interfere with the sample analysis.
Cost-Effective Operations: The long-lasting nature of our solvent filters means fewer replacements, translating to cost savings and uninterrupted laboratory operations.
Pressure-Tolerant Construction: Each filter is built to withstand the high pressures typical of HPLC systems, ensuring consistent performance and safety.
Wide Range of Pore Sizes: Catering to various analytical needs, our solvent filters come in a multitude of pore sizes, affording flexibility in their application across different types of HPLC analyses.
Customization Options: Understanding that different laboratories have unique requirements, we offer customizable filter solutions to perfectly align with your specific analytical demands.
Contact Us :
Ensure the fidelity of your HPLC results with solvent filters from uHPLCs. Take the first step towards unparalleled solvent purity and enhanced laboratory performance. Contact us for expert advice on the ideal filtration solution tailored to your needs. Reach out to us at sales@uhplcs.com and let our solvent filters be the cornerstone of your precise and accurate HPLC analysis.