I. Introduction
A. Definition of Peak Purity:
Peak purity refers to the degree of separation between two peaks in a chromatogram, which is a graphical representation of the components in a sample as they elute from a column in high-performance liquid chromatography (HPLC).
B. Importance of Peak Purity in HPLC:
Peak purity is critical in HPLC because it provides an accurate representation of the sample’s composition. When peaks are not pure, the results of an analysis may be inaccurate, leading to incorrect conclusions and decisions.
C. Purpose of the article:
The purpose of this article is to provide a comprehensive overview of peak purity in HPLC, including its definition, the factors that can affect it, methods for calculating it, and best practices for ensuring peak purity.
II. Understanding the basics of HPLC
A. Definition of HPLC:
HPLC is a liquid chromatography used to separate, identify, and quantify the components in a sample.
It is one of the most widely used analytical techniques in chemistry, biology, and pharmaceuticals.
B. How HPLC works:
HPLC works by passing a sample through a column that contains a stationary phase. The stationary phase is a material that interacts with the sample components and slows them down, causing them to elute at different times. The eluted components are then detected by a detector, such as a UV/Vis spectrophotometer.
C. Column selection:
The choice of a column is critical in HPLC, as it can greatly impact the separation and peak purity of the sample components. Columns come in various lengths, particle sizes, and stationary phase materials, and the appropriate column for a given analysis will depend on the sample and the components being analyzed.
D. Sample preparation:
Sample preparation is another important factor in HPLC, as it can impact the purity of the peaks. Sample preparation typically involves dilution, filtration, or centrifugation to remove any particulate matter or other contaminants that could interfere with the analysis.
III. Factors affecting Peak Purity
A. Column Overloading:
Column overloading occurs when too much sample is introduced into the column, resulting in reduced peak separation and decreased peak purity. It can be caused by introducing too much sample, using a column with too small particle size, or using a column that is not appropriately packed.
B. Column Aging:
Column aging refers to the gradual deterioration of the stationary phase over time, which can result in decreased peak separation and reduced peak purity. It can be caused by repeated use, exposure to high temperatures, or exposure to aggressive mobile phases.
C. Mobile Phase Composition:
The composition of the mobile phase, the liquid used to elute the sample components from the column, can greatly impact peak purity. The pH, buffer concentration, and must carefully choose solvent composition to ensure optimal separation of the sample components.
D. Sample Matrix:
The sample matrix refers to the background of the sample, including any contaminants or impurities that may interfere with the analysis. A complex sample matrix can result in reduced peak separation and decreased peak purity.
IV. Methods for calculating Peak Purity
A. Area Method:
The area method involves measuring the areas of the peaks in the chromatogram and calculating the ratio of the areas of the main peak and any impurities. The result is expressed as a percentage, with 100% indicating a pure peak.
B. Height Method:
The height method involves measuring the heights of the peaks in the chromatogram and calculating the ratio of the heights of the main peak and any impurities. The result is expressed as a percentage, with 100% indicating a pure peak.
C. Relative Retention Method:
The relative retention method involves comparing the retention times of the main peak and any impurities to determine their relative amounts. The result is expressed as a percentage, with 100% indicating a pure peak.
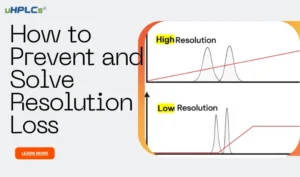
D. Resolution Method:
The resolution method involves calculating the separation between two peaks in the chromatogram. The result is expressed as a number, with higher values indicating better peak separation and greater peak purity.
V. Best practices for Peak Purity in HPLC
A. Proper Column Maintenance:
Regular column maintenance, including cleaning and storage, can help ensure that the column is functioning optimally and provide high-quality separations.
B. Optimization of Mobile Phase Composition:
The composition of the mobile phase should be optimized for each analysis to ensure optimal separation and peak purity. It may involve adjusting the pH, buffer concentration, and solvent composition.
C. Sample Preparation:
Proper sample preparation ensures accurate results and high-quality separations. Careful sample preparation can help remove contaminants and improve the purity of the peaks.
D. Use of Appropriate Columns:
The choice of the column is critical in HPLC, as it can greatly impact the separation and peak purity of the sample components. The appropriate column for a given analysis will depend on the sample and the analyzed components. Choosing a column that provides the necessary separation and peak purity is important.
VI. Conclusion
A. Recap of the Importance of Peak Purity in HPLC:
Peak purity is critical in HPLC because it provides an accurate representation of the sample’s composition. When peaks are not pure, the results of an analysis may be inaccurate, leading to incorrect conclusions and decisions.
B. Final Thoughts:
Ensuring peak purity in HPLC requires careful consideration of various factors, including column maintenance, mobile phase composition, sample preparation, and column selection. Researchers can achieve high-quality separations and accurate results by following best practices and optimizing these factors.
C. Further Reading:
For those interested in learning more about peak purity in HPLC, numerous resources are available, including books, journal articles, and online tutorials.
VII. References A. List of sources used for the article:
-
- Snyder, L. R., & Kirkland, J. J. (2010). Introduction to modern liquid chromatography (3rd ed.). John Wiley & Sons.
-
- Liang, Y. (2010). Chromatography theory and practice. John Wiley & Sons.
-
- Swartz, M. (2002). The basics of chromatography. ASM Press.
-
- Deyl, Z. (2007). Liquid chromatography in the analysis of food and beverages. John Wiley & Sons.
-
- Yang, X. (2006). HPLC and CE principles and practices. CRC Press.