What is Tailing Peaks in HPLC System ?
High-Performance Liquid Chromatography (HPLC) is a versatile analytical technique used to separate, identify, and quantify components in a mixture. This technique is indispensable in various fields, including pharmaceuticals, environmental science, biochemistry, and food analysis. HPLC involves the passage of a liquid sample through a stationary phase packed in a column. The components of the sample interact differently with the stationary phase, leading to their separation based on factors like polarity, size, and charge.
Tailing peaks are a common phenomenon in HPLC, where the trailing edge of a peak is elongated, resulting in a broader and less symmetrical peak shape. This can significantly impact the accuracy of quantitative analysis. Tailing peaks can arise from several factors, including:
- Overloading: Injecting too much sample can saturate the stationary phase, leading to tailing.
- Strong interactions: If the analyte has strong interactions with the stationary phase, it can be retained longer, resulting in tailing.
- Column degradation: Deterioration of the column packing or the stationary phase can cause tailing.
- Incorrect mobile phase conditions: Using a mobile phase that is not optimized for the separation can contribute to tailing.
Understanding the causes of tailing peaks is crucial for optimizing HPLC methods and ensuring accurate analytical results.

Why Causes of HPLC Tailing Peaks Matter
As We Know, there are two reasons cause of HPLC Tailing peak, understanding the causes of HPLC tailing peaks is crucial for analysts aiming to achieve accurate and reproducible results. Tailing peaks can lead to misinterpretation of data, impacting quantitative analysis and overall method reliability.
By identifying the underlying causes, you can implement targeted solutions to enhance the performance of your HPLC system.
Chemical Causes
1.Sample Characteristics:
- pH: The pH of the sample affects the ionization state of analytes, influencing their interactions with the stationary phase. For example, acidic analytes may tail in a reversed-phase column at high pH levels.
- Polarity: The polarity of the analyte relative to the stationary phase is significant; analytes that are too polar or nonpolar can exhibit tailing.
2.Interaction with Stationary Phase:
- Strong Interactions: Analytes with strong interactions with the stationary phase can be retained longer, leading to tailing. This can result from hydrogen bonding, dipole-dipole interactions, or hydrophobic interactions.
Instrumental Causes
1.Column Issues:
- Column Packing: Poor packing can create dead spaces or channels, causing tailing.
- Column Length: Longer columns can increase retention times and the potential for tailing, particularly for analytes with strong interactions.
2.Detector Settings:
- Detector Sensitivity: An overly sensitive detector may amplify noise, resulting in broader peaks and tailing.
- Detector Response Time: A slow response time from the detector can also contribute to tailing.
- Mobile Phase Flow Rate: A high flow rate can decrease retention times but may increase tailing, especially for analytes with strong interactions.
By understanding these causes, analysts can optimize HPLC methods to minimize tailing peaks, ultimately improving the accuracy and precision of their analyses.
Identifying Tailing Peaks
Visual Inspection:
- Asymmetrical Peak Shape: Tailing peaks are characterized by a longer trailing edge compared to the leading edge. This results in a skewed peak shape.
- Broader Peaks: Tailing peaks are often broader than symmetrical peaks, leading to decreased resolution and potentially overlapping peaks.
Quantitative Analysis:
- Tailing Factor: The tailing factor is a quantitative measure of peak asymmetry. It is calculated as the ratio of the distance between the peak maximum and the end of the trailing edge to the distance between the peak maximum and the start of the leading edge. A tailing factor greater than 1 indicates tailing.
Differentiating Tailing from Other Peak Issues:
- Overloading: If the peak is significantly broadened and flattened, it may be due to overloading. Reducing the sample injection volume can help identify if overloading is the cause.
- Detector Noise: Detector noise can lead to broader peaks and increased baseline noise. If the baseline is noisy and the peak shape is not significantly skewed, detector noise is more likely.
- Column Degradation: Column degradation can cause various peak issues, including tailing, broadening, and decreased resolution. If the column has been in use for a long time or has been exposed to harsh conditions, column degradation may be a contributing factor.
By carefully examining chromatograms and using quantitative measures like the tailing factor, analysts can effectively identify tailing peaks and distinguish them from other peak issues. This knowledge is essential for optimizing HPLC methods and ensuring accurate analytical results.
Consequences of Tailing Peaks
Impact on Quantitative Analysis
- Inaccurate Quantification: Tailing peaks can lead to underestimation of the analyte concentration, especially if the peak overlaps with other components in the mixture. This is because the tailing portion of the peak may not be fully integrated, resulting in a reduced peak area.
- Reduced Sensitivity: Tailing peaks can decrease the sensitivity of the analysis, as the signal-to-noise ratio may be lower due to the broader peak shape.
Effect on Method Reproducibility
- Decreased Precision: Tailing peaks can introduce variability into the analysis, leading to decreased precision. This can make it difficult to obtain consistent results, especially when working with low analyte concentrations.
- Reduced Accuracy: Inaccurate quantification due to tailing peaks can also impact the accuracy of the analysis, leading to systematic errors.
To minimize the consequences of tailing peaks, it is essential to optimize HPLC methods and take steps to prevent or reduce tailing. This may involve adjusting the mobile phase composition, modifying the column temperature, or using different column chemistries.
Solutions Trouble Shooting for HPLC Tailing Peaks
HPLC tailing peaks can significantly affect the accuracy and reliability of chromatographic analyses. Identifying and addressing the root causes of tailing is essential for improving peak shapes and overall method performance.
as following 3-solutions guide, we explore effective solutions, including column selection, method optimization, and sample preparation techniques, to help you troubleshoot and minimize tailing peaks in your HPLC workflows, hope it will be helpful for your HPLC Tailing peak troubleshooting.
1. Column Selection and Maintenance
Choosing the Right Column Type:
Select a column with a stationary phase compatible with your analytes. For instance, reversed-phase columns are ideal for separating nonpolar or moderately polar analytes. Consider specialized columns like the Ghost Buster Column from uHPLCs, designed to minimize tailing through advanced technology.
Regular Maintenance Practices:
- Column Cleaning: Periodically clean the column to remove contaminants that can lead to tailing. Always follow the manufacturer’s cleaning guidelines.
- Column Storage: Store the column properly to prevent degradation and contamination.
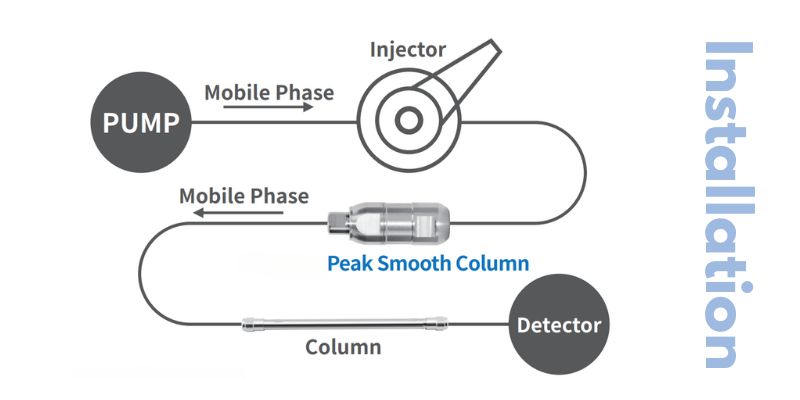
- Column Replacement: Replace the column if it shows significant degradation or contamination. The Peak Smooth Column, also from uHPLCs, can provide improved peak shape and reduce tailing.
2. Method Optimization
Adjusting Mobile Phase Composition:
- Organic Modifier: Increasing the percentage of organic modifier in the mobile phase can decrease retention times and reduce tailing for certain analytes.
- pH: Adjust the pH of the mobile phase to optimize the ionization state of the analytes.
Modifying Temperature and Flow Rates:
- Temperature: Raising the temperature can reduce retention times and improve peak shape for some analytes.
- Flow Rate: Lowering the flow rate can enhance peak shape for analytes with strong interactions with the stationary phase.
3. Sample Preparation Techniques
- Sample Filtration: Filter samples to remove particulate matter that may clog the column and cause tailing.
- Sample Dilution: Dilute concentrated samples to avoid overloading the column.
- Sample Derivatization: For specific analytes, derivatization can enhance peak shape and sensitivity.
By carefully considering these factors and implementing appropriate solutions, including utilizing specialized columns like the Ghost Buster and Peak Smooth Columns from uHPLCs, analysts can effectively mitigate tailing peaks and improve the accuracy and precision of their HPLC analyses.
Case Studies
Real-Life Examples of Tailing Peaks and Resolutions:
1.Case Study 1: Pharmaceutical Analysis
Issue: A pharmaceutical lab noticed tailing peaks in the analysis of a key active ingredient.
Resolution: By adjusting the pH of the mobile phase and switching to a more suitable reversed-phase column, they significantly improved peak shape and reduced tailing.
2.Case Study 2: Environmental Testing
Issue: An environmental testing facility faced tailing peaks when analyzing water samples for contaminants.
Resolution: Implementing a rigorous sample filtration process and optimizing flow rates helped eliminate tailing, leading to more accurate results.
3.Case Study 3: Food Quality Control
Issue: A food testing lab experienced tailing peaks when testing for preservatives in fruit juices.
Resolution: They introduced sample dilution and derivatization techniques, which improved peak shape and overall method sensitivity.
Conclusion
In summary, understanding the causes of HPLC tailing peaks and implementing targeted solutions—such as selecting the right column, optimizing mobile phase composition, and refining sample preparation techniques—can greatly enhance the accuracy and precision of analyses. By proactively addressing these issues, analysts can ensure reliable results in their work.
If you’re facing challenges with your HPLC methods, feel free to reach out for consultation on troubleshooting and optimizing your processes.
Let’s improve our analytical practices together!











