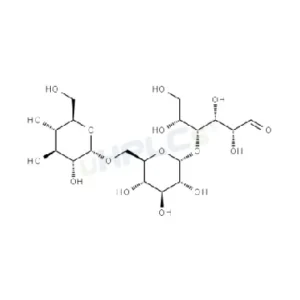
Panose Reference Standard | CAS 33401-87-5
Panose Reference Standard | CAS 33401-87-5 | High-Purity Reference Material Product Code: P-C25037Z Chemical Name: Panose Category: Carbohydrate Standard CAS Number: 33401-87-5 Molecular Formula: /
Home » Portfolio Items » Sucrose Standard | CAS 57-50-1
✅ Ultra-high purity (≥99%) for precise analytical performance
✅ Supplied with Certificate of Analysis (COA) and Safety Data Sheet (SDS) for full traceability
✅ Stable at room temperature with excellent shelf life
✅ Compatible with HPLC, GC, LC-MS, and UV spectrophotometry
✅ Flexible packaging options, including custom sizes for different testing needs
✅ Non-hazardous classification for simplified international shipping
Food & Beverage Analysis
*Determining sugar content in beverages, confectionery, and processed foods.
*Nutritional labeling compliance for global regulatory standards (FDA, EFSA, etc.).
Pharmaceutical Quality Control
*Excipient purity testing and calibration for sugar-based formulations.
*Stability and method validation for injectable and oral dosage forms.
Analytical Method Development
*Calibration of HPLC, GC, and LC-MS instruments for carbohydrate analysis.
*Precision testing for food safety, drug development, and research laboratories.
Industrial Quality Assurance
*Routine monitoring of raw materials and production processes.
*Supporting large-scale manufacturing with accurate testing references.
| Parameter | Details |
|---|---|
| Product Code | S-C25001Z |
| CAS Number | 57-50-1 |
| Chemical Name | Sucrose |
| Molecular Formula | C₁₂H₂₂O₁₁ |
| Molecular Weight | 342.3 g/mol |
| Purity | ≥ 99% |
| Available Sizes | 100 mg (custom sizes available) |
| Storage Conditions | Room Temperature |
| Category | Carbohydrate Standard |
| Structural Formula | Available upon request |
Supplied with COA (Certificate of Analysis) and SDS (Safety Data Sheet).
Manufactured under ISO/IEC 17025 and GMP standards to ensure global compliance.
Classified as non-hazardous, reducing shipping complexity and costs.
Fully traceable production and testing process for regulatory audits.
High-purity sucrose ensures reliable, consistent calibration and test results.
Regular laboratory-grade sucrose may contain trace impurities or variable moisture content, leading to measurement errors and failed regulatory audits.
Using a certified sucrose standard with ≥99% purity provides:
Accurate HPLC and LC-MS calibration for carbohydrate analysis.
Full traceability for audits and quality system documentation.
Confidence in routine testing for food, pharmaceuticals, and industrial processes.
Sucrose standards are widely used across industries:
Food Safety & Regulatory Compliance:
Confirming sugar content in packaged goods and beverages.
Supporting nutritional labeling in compliance with FDA, EFSA, and other standards.
Pharmaceuticals:
Quality control of sucrose used as a drug excipient or stabilizer.
Stability testing in oral and injectable formulations.
Biochemical Research:
Studying metabolic pathways and sugar-related diseases such as diabetes.
Calibration of laboratory equipment for accurate research data.
Manufacturing & Industrial QC:
Process monitoring and verification during bulk sugar production.
Reference testing for production line validation.
Every sucrose standard shipment includes:
COA (Certificate of Analysis): Provides batch-specific purity, molecular identity, and analytical data.
SDS (Safety Data Sheet): Ensures safe handling, storage, and compliance with workplace safety regulations.
These documents are essential for:
Regulatory inspections by agencies like FDA or EFSA.
Maintaining traceable quality control records.
Ensuring product safety and accuracy for laboratory audits.
Sucrose standards can be used with a variety of testing methods, including:
HPLC (High-Performance Liquid Chromatography): Quantitative sugar analysis in food and pharmaceutical samples.
LC-MS/MS: High-sensitivity identification in complex biological matrices.
GC (Gas Chromatography): For derivatized sugar testing.
UV/Vis Spectrophotometry: Quick and cost-effective sugar concentration measurement.
The high purity of the standard ensures accurate baseline readings and repeatable results across all systems.
Sucrose standards are stable at room temperature. For best results:
Store in a dry environment away from humidity.
Keep containers tightly sealed to prevent contamination.
Avoid direct sunlight and extreme temperature fluctuations.
Following these practices will preserve purity and ensure consistent testing performance over time.
Our sucrose standard is manufactured with a focus on purity, traceability, and compliance:
≥99% purity, validated using advanced analytical methods.
Documentation (COA & SDS) included with every batch.
Production under ISO/IEC 17025 and GMP-certified systems.
Fast global shipping with simplified logistics due to non-hazardous classification.
These advantages make it ideal for laboratories and production environments requiring consistent, reliable reference materials.
📧 Contact Sales: sales@uhplcs.com
📞 Support Hotline: +86-0755-28502380
👉 [Request a Quote Now]

Panose Reference Standard | CAS 33401-87-5 | High-Purity Reference Material Product Code: P-C25037Z Chemical Name: Panose Category: Carbohydrate Standard CAS Number: 33401-87-5 Molecular Formula: /
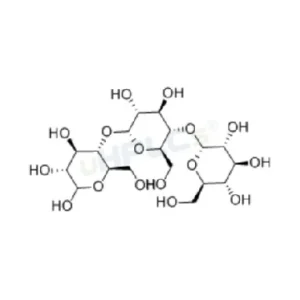
Maltotriose Reference Standard | CAS 1109-28-0 | High-Purity Reference Material Product Code: M-C25036Z Chemical Name: Maltotriose Category: Carbohydrate Standard CAS Number: 1109-28-0 Molecular Formula: /
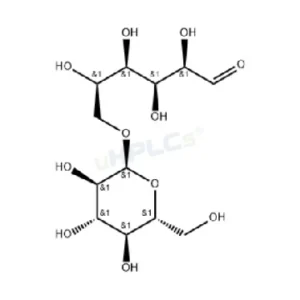
Lactulose Reference Standard | CAS 4618-18-2 | High-Purity Reference Material Product Code: L-C25034X Chemical Name: Lactulose Category: Carbohydrate Standard CAS Number: 4618-18-2 Molecular Formula: C₁₂H₂₂O₁₁
WhatsApp us
Subscribe for exclusive offers and updates on new arrivals